Details of the Drug
General Information of Drug (ID: DM1F42Z)
| Drug Name |
Oxatomide
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Celtect; Oxatimide; Oxatomida; Oxatomidum; Oxetal; Tinset; CBMicro_024634; KW 4354; O 9387; R 35443; R35443; Celtect (TN); KW-4354; McN-JR 35443; Oxatomida [INN-Spanish]; Oxatomide (tinset); Oxatomidum [INN-Latin]; R 35,443; R-35443; Oxatomide (JAN/USAN/INN); Oxatomide [USAN:BAN:INN:JAN]; 1-(3-(4-(Diphenylmethyl)-1-piperazinyl)propyl)-1,3-dihydro-2H-benzimidazol-2-one; 1-(3-(4-(Diphenylmethyl)-1-piperazinyl)propyl)-2-benzimidazolinone; 1-[3-(4-Benzhydryl-piperazin-1-yl)-propyl]-1,3-dihydro-benzoimidazol-2-one; 1-[3-[4-(Diphenylmethyl)-1-piperazinyl]propyl]-1,3-dihydro-2H-benzimidazol-2-one; 1-[3-[4-(Diphenylmethyl)-1-piperazinyl]propyl]-2-benzimidazolinone; 3-[3-(4-benzhydrylpiperazin-1-yl)propyl]-1H-benzimidazol-2-one
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antiallergic Agents
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
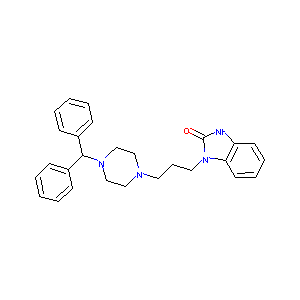 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 426.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Hay fever | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | CA08.00 | |||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||
References
